

India

South Africa

Japan
Purisol
All extracted from the reference: below.
Higman C & G. Gruenfelder: “Clean Power Generation From Heavy Residues Clean Power Generation", presented at the Conference “Power Generation and the Environment”, Institution of Mechanical Engineers, Art. 3.2, London, November (1990)
There is a wide variety of solvents available for desulphurization of the raw gas. When selecting the optimum solvent for an IGCC plant there are a number of important factors to consider.
(a) Selectivity.
All desulphurization solvents absorb a certain amount of CO2. In many circumstances this is of no great importance, but to the IGCC co-absorption represents a loss of mass flow to the gas turbine as well as inflating the cost of the Claus and recompression sections. A high selectivity between H2S and CO2 is therefore required.
(b) Carbonyl Sulphide.
In general one finds that the solubility of carbonyl sulphide (COS) is substantially lower than that of H2S and is thus the limiting factor in the desulphurization process. For high sulphur recovery rates it is therefore necessary to look for a solvent with a high COS solubility or introduce an additional catalytic process stage to remove the COS.
The solvent of LURGI's PURISOL desulphurization process, N-methyl-2pyrrolidone (NMP) meets these two basic requirements. Over the last few years (1088-1990/SAFE comment) the process has been further optimized specifically for the IGCC application
Complementing the Purisol washing system, a Claus unit converts the gaseous sulphur compounds in the Purisol waste gas to elemental sulphur. In LURGI's optimized flow scheme the Claus tail gas is recycled to the Purisol wash thus eliminating completely any sulphur emissions from the Claus unit and simultaneously removing the need for separate tail gas treatment.
The basic outline of the Purisol desulphurization unit is shown in Figure 5.
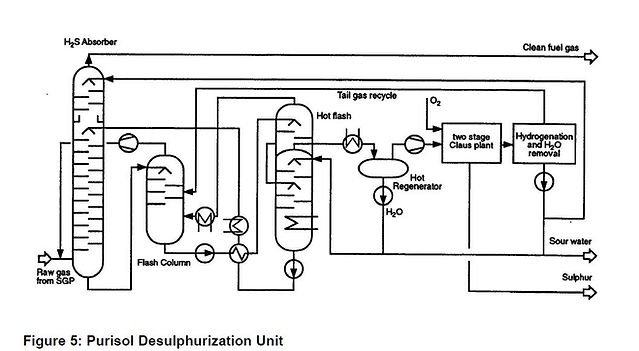
The raw gas from the SGP unit is washed with NMP in the H2S absorber. The NMP absorbs the H2S from the raw gas. By hydrolysing the COS to H2S the NMP also indirectly absorbs the COS. The gas leaves the absorber with a residual H2S plus COS content of typically 20 - 40 ppmv. Lower values can be achieved with only a minor increase in capital cost.
The loaded NMP undergoes an intermediate flash at a pressure of about 2 bar releasing most of the coabsorbed CO2, CO and H2. This flash gas is recompressed to the absorber. The NMP is subjected to a further hot flash and hot regeneration before being recycled to the absorber.
SAFE's note:
Since the website of Air Liquide, who aquired Lurgi, do not list the Purisol process in the list of available technologies, the process may not be licensed any more.